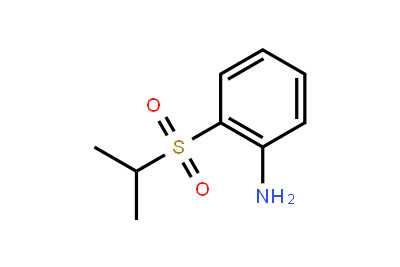
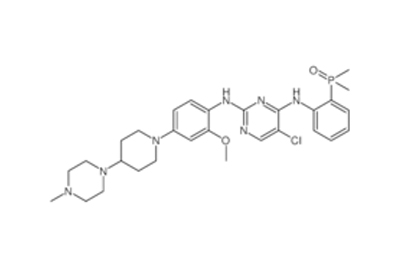
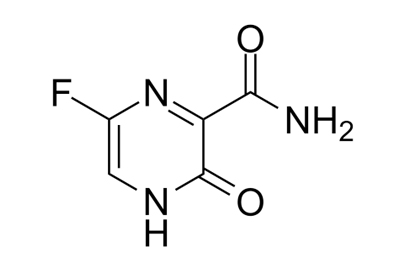
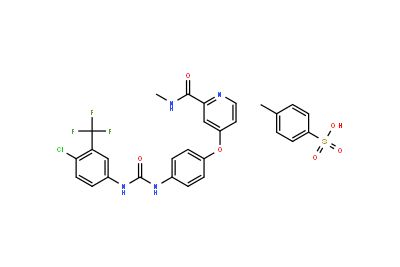
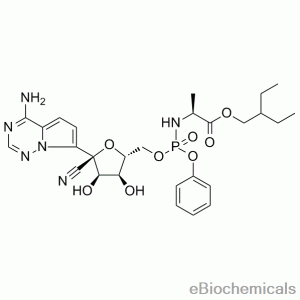
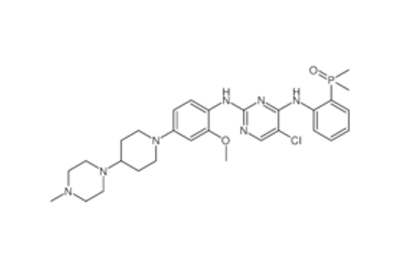
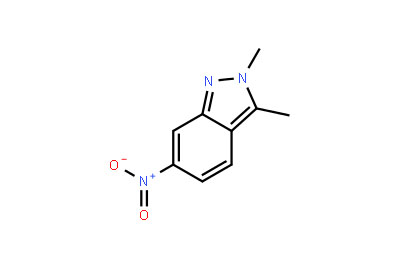
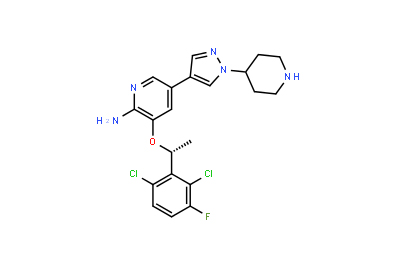
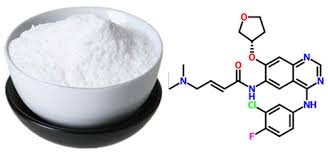
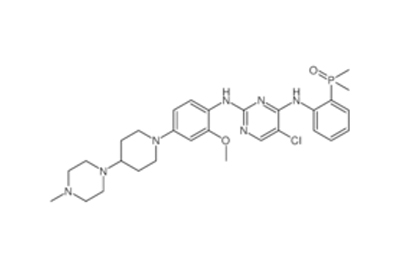
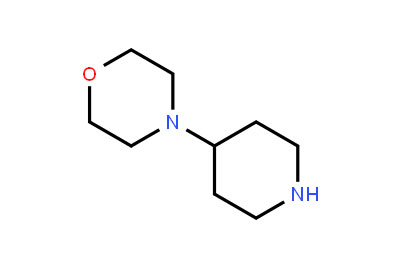
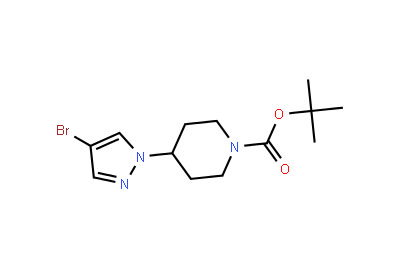
Drug intermediates from Intermediate Manufacturer can refer to a substance created during the synthesis of an API that needs to go through additional molecular processing or modification before it is an API. Drug intermediates are the medications utilized as raw materials for the creation of bulk pharmaceuticals. For the pharmaceutical and cosmetic sectors, high-quality raw components are manufactured hygienically into drug intermediates.

Pharmaceutical companies use drug intermediates in their research and development efforts. Pharmaceutical intermediates, veterinary intermediates, and bulk intermediates are a few examples of several types of drug intermediates. The increase in global R&D efforts is expected to result in a rapid expansion of the global market for pharmaceutical intermediates.
Regions, end users, and different intermediate types are segmented on the market from Intermediate Supplier China.
The market is divided into two main categories based on the kind of intermediate: advanced intermediate and API intermediate. The basic materials or raw pharmaceuticals that are responsible for the therapeutic activity are known as APIs, or active pharmaceutical ingredients. These are the active ingredients that are later changed into various forms, such as pills, capsules, solutions, etc.
Even API functions as a drug. Drug intermediates can also be found in advanced forms. Advanced intermediate medicines are used to carry out drug interaction activity developed in unique chemical compounds. A few examples of drug intermediates are gemcitabine, imatinib, lenalidomide, pemetrexed, nilotinib, temozolamide, pazopanib, and ibrutinib intermediates.

The pharmaceutical, biotechnology, and chemical industries are the three subsectors under which the worldwide market for drug intermediates is divided according to end user. There are several different types of medication intermediates, including low quality intermediates, high quality intermediates, and premium grade intermediates. The high- and premium-quality medication intermediates are mostly utilized for academic research.
Due to the rapidly expanding biotechnology and life sciences industries, rising acceptance, and expanding use of drug intermediates in research disciplines, there has been an ever-increasing demand for drug intermediates on the global market. Producers have produced exquisite chemicals for pharmaceutical firms and Intermediate Manufacturer China. We are able to meet market needs thanks to their expanding production capacity and solid market position.
Follow us on Twitter