Cancer is one of the leading causes of death globally and has affected millions of people worldwide. The search for a cure and a more effective cancer treatment has been a continuous process for years, and with the advancement of technology, breakthroughs are being made every day. One such breakthrough is Lenvatinib Intermediate.
Lenvatinib is a type of kinase inhibitor, a medication used in the treatment of cancer. It works by blocking the activity of specific proteins in cancer cells, which can slow down or stop the growth of these cells. Lenvatinib is approved for the treatment of several types of cancers, including thyroid cancer and endometrial cancer. This drug is considered a breakthrough because of its ability to target multiple pathways in cancer cells, making it more effective than traditional chemotherapy drugs.
Lenvatinib Intermediate works by blocking the activity of specific proteins that are involved in the growth and spread of cancer cells.
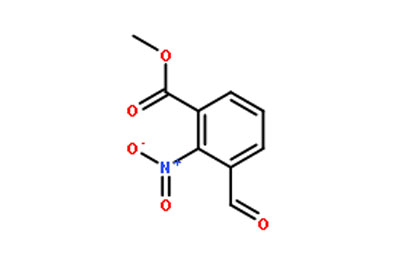
Lenvatinib intermediate is an intermediate stage in the manufacturing process of Lenvatinib. In this stage, the raw materials used to make the drug are converted into an intermediate product that is then processed further to create the final drug. The intermediate stage is critical in ensuring the quality and purity of the final product.
One of the challenges in the production of Lenvatinib intermediate is controlling the reaction conditions to ensure that the correct product is formed. This requires careful attention to detail and a high level of expertise in the field of pharmaceutical manufacturing. You can Buy Lenvatinib Intermediate online.
The quality of the Lenvatinib intermediate product is crucial, as it affects the final drug’s efficacy and safety. The intermediate stage is subject to strict quality control procedures to ensure that the product meets the required standards.
In conclusion, the Lenvatinib intermediate is a critical stage in the manufacturing process of Lenvatinib. The quality of the intermediate product directly affects the quality of the final drug, and strict quality control procedures are in place to ensure that the product meets the required standards. If you are taking Lenvatinib Intermediate or considering taking it, it is important to understand the role of the intermediate stage in the drug’s production and how it affects the final product.
Follow us on Twitter